
PROPERTIES OF ACIDS
- Corrosive ('burns' your skin)
- Sour taste (e.g. lemons, vinegar)
- Contains hydrogen ions (H+) when dissolved in water
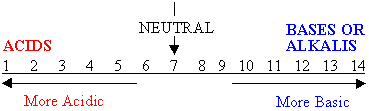
- Has a pH less than 7
- Turns blue litmus paper to a red colour
- Reacts with bases to form salt and water
- Reacts with metals to form hydrogen gas
- Reacts with carbonates to form carbon dioxide, water and a salt
EXAMPLES OF ACIDS
- Hydrochloric acid (HCl) in gastric juice
- Sulphuric acid (H2SO4)
- Nitric acid (HNO3)
- Carbonic acid in softdrink (H2CO3)
- Uric acid in urine
- Ascorbic acid (Vitamin C) in fruit
- Citric acid in oranges and lemons
- Acetic acid in vinegar
- Tannic acid (in tea and wine)
- Tartaric acid (in grapes)
PROPERTIES OF BASES AND ALKALIS
- Corrosive ('burns' your skin)
- Soapy feel
- Has a pH more than 7
- Turns red litmus paper to a blue colour
- Many alkalis (soluble bases) contain hydroxyl ions (OH-)
- Reacts with acids to form salt and water
EXAMPLES OF BASES AND ALKALIS
- Sodium hydroxide (NaOH) or caustic soda
- Calcium hydroxide ( Ca(OH)2 ) or limewater
- Ammonium hydroxide (NH4OH) or ammonia water
- Magnesium hydroxide ( Mg(OH)2 ) or milk of magnesia
- Many bleaches, soaps, toothpastes and cleaning agents
THE pH SCALE
Common Acids |
pH |
Common Bases |
pH |
| Hydrochloric acid Sulphuric acid Stomach juice Lemons Vinegar Apples Oranges Grapes Sour milk White bread Fresh milk |
0.1 0.3 1-3 2.3 2.9 3.1 3.5 4 4.4 5.5 6.5 |
Human saliva Distilled water Blood plasma Eggs Seawater Borax Milk of magnesia Ammonia water Limewater Caustic soda |
6-8 7 7.4 7.8 7.9 9.2 10.5 11.6 12.4 14 |
INDICATORS
An indicator, when added to an acid, a neutral substance or a base, will change different colours.
| INDICATOR | COLOUR IN ACID | COLOUR IN NEUTRAL SOLUTION | COLOUR IN BASE |
| Litmus | red | purple | blue |
| Bromothymol Blue | yellow | blue | blue |
| Phenolphthalein | clear | clear | purple |
| Universal Indicator | red | Yellow-green | purple |
REACTIONS WITH ACIDS
| ACID | + | BASE | SALT | + | WATER | |
| Hydrochloric Acid | + | Sodium Hydroxide | Sodium Chloride | + | Water | |
| HCl | + | NaOH | NaCl | + | H2O |
| ACID | + | METAL | SALT | + | HYDROGEN GAS | |
| Hydrochloric Acid | + | Magnesium | Magnesium Chloride | + | Hydrogen | |
| HCl | + | Mg | MgCl2 | + | H2 |
| ACID | + | CARBONATE | SALT | + | WATER | + | CARBON DIOXIDE GAS | |
| Hydrochloric Acid | + | Calcium Carbonate | Calcium Chloride | + | Water | + | Carbon Dioxide | |
| HCl | + | CaCO3 | CaCl2 | + | H2O | + | CO2 |