
AN ATOM
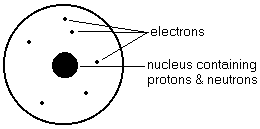
- Atom - smallest unit of all matter, that is composed of 3 sub-atomic particles called protons, electrons and neutrons
- Proton - the 'heavy' positively-charged particle in the nucleus of an atom
- Electron - the very 'light' negatively-charged particle that orbits the nucleus of an atom
- Neutron - the 'heavy' uncharged particle in the nucleus of an atom
- Uncharged or unreacted atoms have the same number of positive protons and negative electrons.
- Approximate size of atoms - Millions of atoms could fit on the sharp point of a needle. Also, if you imagine that an atom is the size of an oval, a proton and a neutron would be the size of footballs in the middle of the oval, and the electron would be the size of a rice grain racing around the running lane.
ATOMIC NUMBER AND ATOMIC MASS
- An Example from the Periodic Table
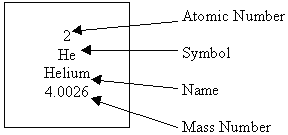
- Atomic Number - the number of protons in an unreacted atom
- Mass Number - the number of protons and neutrons together
ATOMIC DIAGRAMS
- Protons and neutrons are in the nucleus.
- Protons are p+
- Neutrons are n
- Electrons orbit the nucleus in electron levels or 'rings'.
- Electrons are e-
- A limited number of electrons are situated in each electron 'ring'.
- First ring - maximum of 2 electrons
- Second ring - maximum of 8 electrons
- Third ring - maximum of 8 electrons
- Fourth ring - maximum of 18 electrons
- Electron rings close to the nucleus are filled first.
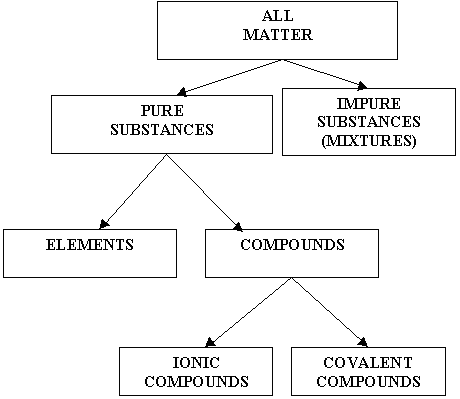
ELEMENTS AND COMPOUNDS
- Element - An element is a substance composed of the same type of atoms (e.g. gold Au, oxygen O2).
- Compound - A compound is a substance made of more than one type of atom (e.g. water H2O, carbon dioxide CO2).
- Molecule - A molecule is the smallest particle of either an element or a compound.
INERT OR NOBLE GASES
- Inert or Noble Gases are unreactive gases. They do not corrode nor react.
- Examples of Noble Gases are:
- He - Helium
- Ne - Neon
- Ar - Argon
- Kr - Krypton
- Xe - Xenon
- Rn - Radon
- The electron rings of these unreactive gases are full, therefore they become stable.
IONS (CHARGED ATOMS)
- When atoms react, they may either gain or lose electrons. Electrons have a negative charge. An atom gaining or losing electrons will get an overall charge.
- Positive Ions are atoms that have lost electrons (e.g. sodium Na1+)
- Negative Ions are atoms that have gained electrons (e.g. chlorine Cl1-)
- In chemical reactions, atoms tend to gain or lose electrons to resemble the electron numbers of the stable Noble Gases.
COVALENT AND IONIC COMPOUNDS
- Covalent Compound - a compound where electrons are shared between the atoms (e.g. carbon dioxide CO2)
- Ionic Compound - a compound formed from the attraction between positive and negative ions. For example in the ionic compound sodium chloride NaCl, the chlorine ion (Cl1-) gains one electron that was given by the sodium ion (Na1+).
COMMON ELEMENTS AND SYMBOLS TO LEARN
Element Symbol |
Element Name |
Element Symbol |
Element Name |
| H | Hydrogen | Mn | Manganese |
| He | Helium | Fe | Iron |
| Li | Lithium | Co | Cobalt |
| C | Carbon | Ni | Nickel |
| N | Nitrogen | Cu | Copper |
| O | Oxygen | Zn | Zinc |
| F | Fluorine | Br | Bromine |
| Ne | Neon | Ag | Silver |
| Na | Sodium | Sn | Tin |
| Mg | Magnesium | I | Iodine |
| Al | Aluminium | Ba | Barium |
| Si | Silicon | W | Tungsten |
| P | Phosphorus | Pt | Platinum |
| S | Sulphur / Sulfur | Au | Gold |
| Cl | Chlorine | Hg | Mercury |
| Ar | Argon | Pb | Lead |
| K | Potassium | Cr | Chromium |
| Ca | Calcium | Ti | Titanium |
| Pu | Plutonium | U | Uranium |
NAMING COMPOUNDS
PREFIX OR |
MEANING |
EXAMPLE |
| Mono- | There is 1 atom of that type in that molecule | Carbon monoxide (CO) |
| Di- | There are 2 atoms of that type in the molecule | Carbon dioxide (CO2) |
| Bi- | Hydrogen is present in the molecule | Sodium bicarbonate (NaHCO3) |
| -ide | There are only 2 types of atoms present in the molecule | Lead oxide (PbO) |
| -ate | There are 3 or more types of atoms in the molecule, and 1 type is oxygen | Calcium carbonate (CaCO3) |
VALENCY TABLE
- Valency - the charge of an ion or radical which has either lost or gained electrons
- Note that metals lose electrons easily to become positive ions. This is why most metals are good conductors of electricity.
1+ |
2+ |
3+ |
1- |
2- |
3- |
| H 1+ | Mg 2+ | Al 3+ | F 1- | O 2- oxide |
PO4 3- phosphate |
| Na 1+ | Ca 2+ | Fe 3+ ferric |
Cl 1- | S 2- sulphide |
|
| Li 1+ | Cu 2+ | Br 1- | CO3 2- carbonate |
||
| K 1+ | Zn 2+ | OH 1- hydroxide |
SO4 2- sulphate |
||
| Ag 1+ | Pb 2+ | NO3 1- nitrate |
|||
| NH4 1+ ammonium |
Fe 2+ ferrous |
HCO3 1- bicarbonate |
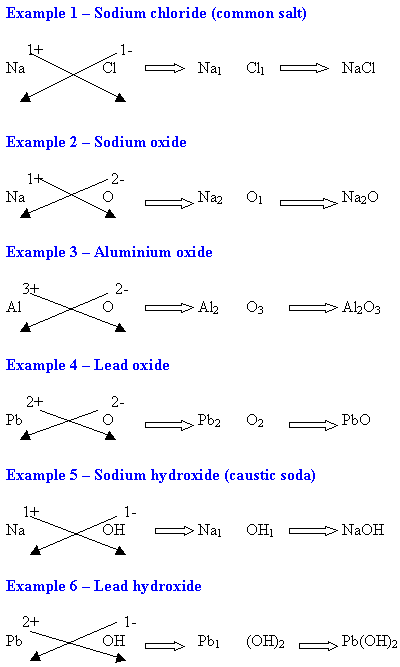
WORKING OUT FORMULAE OF IONIC COMPOUNDS
(THE CROSS-OVER METHOD)
- Step 1 - In the ionic compounds to be learnt in junior science, there are two parts to the ionic compound - the first is a positive ion (usually a metal e.g. Na1+) and the second is a negative ion (e.g. Cl1-).
- Step 2 - Using the valency table, write the two ions and their valencies.
- Step 3 - Now ignore the positive and negative signs. Cross-over the top valency number to the bottom of the other ion symbol. Do this for both.
- Step 4 - Write the completed formulae with those same numbers at the bottom.
- Step 5 - If the numbers on each part are the same (e.g. Na1 Cl1 or Mg2 O2), ignore them and rewrite the formulae without them (e.g. Na Cl or Mg O).
- Step 6 - Brackets may be used around radicals (groups of atoms that are charged e.g CO3).
EXAMPLES OF CHEMICAL NAMES OF COMPOUNDS
CHEMICAL FORMULA |
CHEMICAL NAME |
| CO2 | carbon dioxide |
| CO | carbon monoxide |
| Na Cl | sodium chloride |
| Cu O | copper oxide |
| Ag Br | silver bromide |
| K I | potassium iodide |
| H Cl | hydrogen chloride (hydrochloric acid) |
| NH4 Cl | ammonium chloride |
| K OH | potassium hydroxide |
| Na OH | sodium hydroxide |
| Ca (OH)2 | calcium hydroxide |
| Ca S | calcium sulphide |
| Na NO3 | sodium nitrate |
| H NO3 | hydrogen nitrate (nitric acid) |
| Na HCO3 | sodium bicarbonate |
| Zn SO4 | zinc sulphate |
| Mg CO3 | magnesium carbonate |
| Ca SO4 | calcium sulphate |
| Cu CO3 | copper carbonate |
| Al PO4 | aluminium phosphate |
| Fe SO4 | iron sulphate |
| Fe CO3 | iron carbonate |
| NH4 NO3 | ammonium nitrate |
| NH4 HCO3 | ammonium bicarbonate |
| H2 SO4 | hydrogen sulphate (sulphuric acid) |
| Na2 SO4 | sodium sulphate |
| (NH4)2 CO3 | ammonium carbonate |
EXAMPLES OF NUMBERS AND TYPES OF ATOMS
IN VARIOUS ELEMENTS AND COMPOUNDS
NAME OF |
CHEMICAL |
ELEMENT OR COMPOUND |
NUMBER AND TYPE |
| Hydrogen | H2 | element | 2 hydrogen atoms |
| Carbon dioxide | CO2 | compound | 1 carbon atom 2 oxygen atoms |
| Water | H2O | compound | 2 hydrogen atoms 1 oxygen atom |
| Methane | CH4 | compound | 1 carbon atom 4 hydrogen atoms |
| Sodium hydroxide | NaOH | compound | 1 sodium atom 1oxygen atom 1 hydrogen atom |
| Calcium hydroxide | Ca(OH)2 | compound | 1 calcium atom 2 oxygen atoms 2 hydrogen atoms |