
PHYSICAL AND CHEMICAL CHANGES
Burn a Sugar Cube
- Safety Rules:
- Parent supervision
- Take care with fire or heat
- Do it outdoors
- Materials you need are:
- 3 sugar cubes (from the supermarket)
- powdered carbon (e.g. remnants of a campfire, cigarette ash)
- matches
Try burning the sugar cube with the lit match first. It shouldn't burn.
Now dip the sugar cube in cigarette ash (carbon) and light it again. It should light much more easily.
The carbon heats up and raises the temperature of the surrounding sugar crystals to the ignition temperature required for the sugar to burn. Once these first crystals are burning, they provide sufficient heat for the neighbouring crystals to continue burning.
Face Paint
- Materials you need are:
- 1/4 cup of water
- food colouring
- 1/2 cup of cornstarch
- 1/4 cup of cold cream
- small brushes
- small container
- plastics spoons
Mix cornstarch, water and cold cream together thoroughly. Spoon into containers, one for each colour. Add food colouring to each container of face paint. Use brushes to paint faces.
This face paint washes of with soap and water.
How Much Oxygen is in Air
- Safety Rules:
- Parent supervision
- Take care with fire or heat
- Materials you need are:
- a large clear container
- a smaller clear container
- water
- a small candle
- a small light dish on which the candle floats
- matches
Set up the materials as in the diagram. Light the candle and put the cover over it last. Wait a few minutes until the candle goes out. Watch the water in the small container rise.
Oxygen takes up almost 20% or one-fifth of air. When all of the oxygen in the container has been used in burning the candle, it will be replaced by the water rising.
Invisible Ink
- Safety Rules:
- Parent supervision
- Take care with fire or heat
- Materials you need are:
- freshly-squeezed lemon juice
- a toothpick or a fine stick as a pen
- a sheet of paper
- a candle
- matches
The 'ink' is the lemon juice. Dip the toothpick or fine stick into the lemon juice and write you name on the paper. Allow it to dry completely. Light the candle and hold the paper above the flame so that heat only burns the lemon juice writing but not the paper.
The ignition temperature needed to burn the sugar in the lemon juice is lower than that of the paper.
Snuff Out a Candle with a Copper Coil
- Safety Rules:
- Parent supervision
- Take care with fire or heat
- Materials you need are:
- thick copper or aluminium wire (about 30cm long)
- a candle
- matches
-
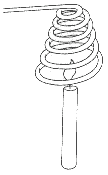
Make a coil of wire with either thick copper or thick aluminium wire. Hold the cold wire coil over a burning candle for a short time. The candle should go out.
The fire is extinguished because the coil of wire conducts heat away from the flame so fast that the temperature below the ignition temperature.