
SOLUBILITY TERMS
- Dissolve - When a substance dissolves, it breaks apart into smaller particles in the solution. These particles cannot be seen with the naked eye.
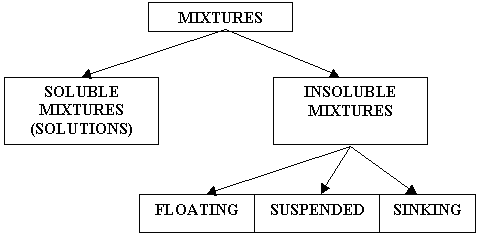
- Solution - A solution is a mixture containing one substance that dissolves (solute), and another substance that does the dissolving (solvent). An example of a solution is salty water.
- Solute - the substance that dissolves (e.g. salt in salty water)
- Solvent - the substance that does the dissolving (e.g. water in salty water)
SOLUTION |
SOLUTE |
SOLVENT |
| Salty water | Salt (solid) | Water (liquid) |
| Cup of instant coffee | Coffee (solid) | Hot water (liquid) |
| Cleaning paint brushes | Paint (liquid) | Turpentine or Water (liquids) |
| Softdrink | Carbon dioxide (gas), sugar and flavours (solids) | Water (liquid) |
| Solder | Tin (solid) | Lead (solid) |
- Solubility - Solubility is the ability of a substance to dissolve. Solids such as salt are more soluble in hot water than in cold water. However, gases such as oxygen and carbon dioxide are more soluble in cold water than in hot water.
- Dilute - A dilute solution is a type of solution where a small amount of solute dissolves in a large amount of solvent (e.g. a cup of instant coffee with little coffee and a lot of hot water)
- Concentrated - A concentrated solution is a type of solution where a large amount of solute dissolves in a small amount of solvent (e.g. a cup of instant coffee with a lot of coffee and very little hot water)
- Saturated - A saturated solution is a type of solution where so much solute has been added to the solvent, that no more can dissolve. This type of solution is needed to grow crystals.
CRYSTALS
- Crystalline Solids are solids that are composed of fixed ordered structures such as cubes (e.g. every solid except glass and some plastics).
- Amorphous Solids are solids that are not crystalline (e.g. glass and some plastics).
- Procedure to Grow Crystals
- Heat 100 mL of water in a beaker, and dissolve as much solid (e.g. blue copper sulphate) as possible, stirring constantly.
- Continue heating and dissolving until no more solid will dissolve. A saturated solution has been made.
- Place a rod with a small crystal suspended on a thread across the top of the beaker. The crystal should be immersed in the saturated solution.
- Observe after several days.
SEPARATION OF MIXTURES
- Decanting - pouring liquid from a mixture containing both liquid and insoluble solid (e.g. pouring the water out of a bucket containing a mixture of mud and water)
- Filtration - separates soluble from insoluble substances (e.g. using a strainer to separate cooked spaghetti from the boiling water)
- Evaporation - heating to evaporate the water from insoluble or soluble particles in the mixture (e.g. collecting salt from seawater by letting the water evaporate)
- Distillation - evaporation followed by condensation (e.g. obtaining pure drinking water from seawater)
- Chromatography - separates coloured dyes by dissolving the dyes in a solvent and drawing the lighter and heavier particles along a filter paper strip
- Using a Centrifuge - uses spinning to separate lighter and heavier particles (e.g. separating the lighter liquid plasma from the heavier blood cells in blood)
- Using a Magnet - attracts substances containing iron, nickel and cobalt